

#METAL REACTIVITY TABLE SERIES#
In order to understand the electrochemical series and how they arranged, first, we will consider the explanation of some other terminologies. With standard electrode potential of hydrogen electrode which is also called

Electrode potential isĪlso reduction potential and it is calculated for many elements by comparing Standard electrode potential under standard conditions. Of the elements or their ions in increasing or decreasing order of their The metal is one of the five metals that can hold the liquid form at room temperature.Electrochemical series is defined as the arrangement Cesium is an alkali metal with a soft silvery-golden tint. The reaction of sodium is more vigorous, whereas the reaction of calcium is less vigorous.Ī. Sodium (Na) and Calcium (Ca) react with cold water. Name Two Metals that react with cold water.Ī. The medal belongs to Group 12 of the periodic table. The metal is applied to galvanize steel to protect the metal from corrosion. Zinc is a base metal that is used in biological and industrial uses. Nickel creates a layer of nickel oxide to stop corrosion. Frequently Asked Questions (FAQs)Ī.Nickel is not at all a reactive metal. The electrons of Xenon are deeply connected with atoms.

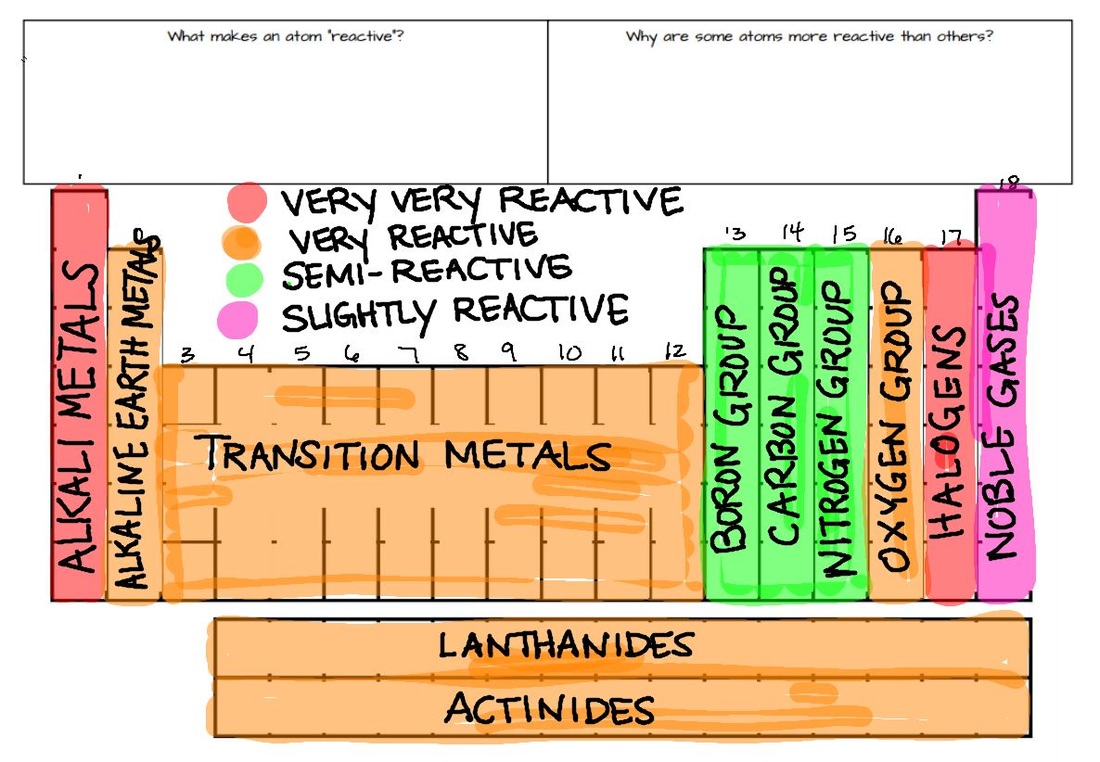
The metals above require a high amount of energy according to their high position.The metals in the higher position can displace metals of the lower position.The electro-positive power of the elements decreases while going downwards the series.The reducing ability lessens as the series goes down.The metals reactive to water are strong in reducing the agents as the metals are easily oxidized.Different metals react differently to oxygen in the presence of other natural elements like air. The reaction of metals with oxygen forms metal oxides. In the reactive process, metals lose electrons and create positive ions. Reactive Series of the Common Metals is mentioned below, The blog will discuss the reactive series through the metal charts in detail, focusing on various essential aspects. The series also offers information on the reactivity of the metals towards acids and water. The reactivity series helps to predict if a metal can change the position of another metal in a single reaction. The series presents the downwards order of various metals based on the strength of their reactions. Another synonymous term of reactivity series is 'activity series'. The Definition of Reactivity series primarily indicates the reaction of metals to various elements.


 0 kommentar(er)
0 kommentar(er)
