
Performing facility name and CLIA number.Ordering provider name and nonpharmaceutical interventions (as applicable).
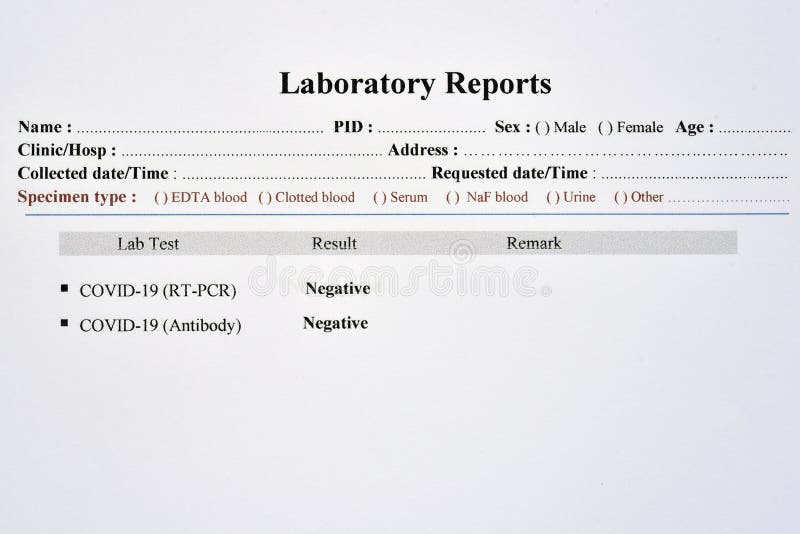
Sample negative covid test results code#
Test result–use appropriate LOINC and SNOMED codes, as defined by the Laboratory In Vitro Diagnostics (LIVD) Test Code Mapping for SARS-CoV-2 Tests provided by CDC. Test ordered – use harmonized LOINC codes provided by CDC. Laboratories should make every reasonable effort to provide the following data elements when reporting to state and jurisdictional health departments. Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any. Test data submitted to NHSN will be reported to appropriate state and local health departments using standard electronic laboratory messages. This CDC- and CMS-preferred pathway to submit data to CDC’s NHSN applies only to CMS-certified long-term care facilities. CMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN). Submit laboratory testing data through a state or regional Health Information Exchange (HIE) to the appropriate state or local public health department. Submit laboratory testing data to state and local public health departments through a centralized platform, where the data will then be routed to the appropriate state and local authorities and may be routed to CDC after removal of personally identifiable information according to applicable rules and regulations. Data must be sent using existing reporting channels to ensure rapid initiation of case investigations, and concurrent reporting of results must be shared with the ordering provider or patient, as applicable. Submit laboratory testing data directly to state or local public health departments according to state/or local law or policy. Laboratory data elements may be reported in the following ways: COVID-19 home tests can be safely and privately reported at. Healthcare providers can ensure that those who have tested positive for COVID-19 receive the most appropriate medical care, including specific treatments if necessary. 
NOTE regarding self-test results: While there are no current mechanisms that require reporting of self-test results to public health authorities, CDC encourages everyone who uses a self-test to report any positive results to their healthcare provider. For more information, see the Center for Medicare and Medicaid Service’s (CMS) Research Testing and Clinical Laboratory Improvement Amendments of 1988 (CLIA) Regulations. If at any time a facility intends to report a patient-specific test result, it must first obtain a CLIA certificate and meet all requirements to perform testing. Testing sites that perform COVID-19 surveillance testing on de-identified samples, regardless of their CLIA status, should not report the results of their surveillance testing to state, tribal, local, and territorial public health departments.
Other types of LTC facilities may also report testing data in NHSN for self-tracking or to fulfill state or local reporting requirements, if any.
other facilities or locations offering COVID-19 point-of-care diagnostic or screening tests, or in-home diagnostic or screening tests.ĬMS-certified long-term care facilities may submit point-of-care SARS-CoV-2 testing data, including antigen testing data, to CDC’s National Healthcare Safety Network (NHSN).non-laboratory COVID-19 diagnostic or screening testing locations, and.laboratories that perform clinical diagnostic or screening testing under CLIA,.follow all state specific-reporting requirements for COVID-19 test results.
meet all requirements to perform testing, including only using FDA-authorized test systems according to their instructions for use, and.have a Clinical Laboratory Improvement Amendments (CLIA) certificate,.








 0 kommentar(er)
0 kommentar(er)
